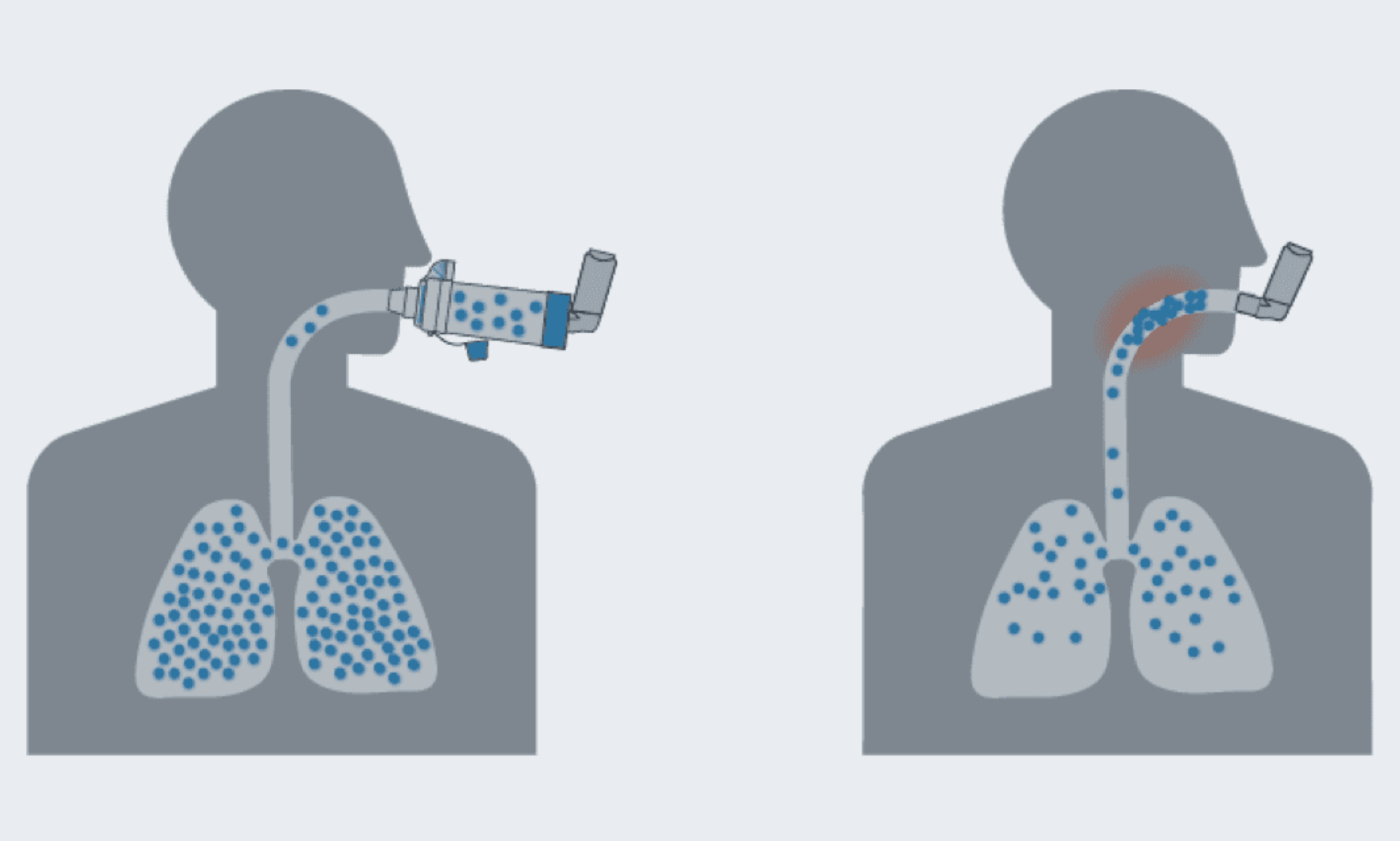
Requirements
Assist patients in achieving an optimal inhalation flow rate.
Specifications:
1.1) Measure flow rate at flow alert port for a minimum of six seconds
1.2) Facilitate an optimal flow rate between 30-60 L/min
Justification: Studies show that inconsistent inhalation affects medication deposition.
Must provide real time visual feedback to help patients maintain optimal inhalation flow and duration
Specifications:
2.1) Feedback mechanism must indicate when patient is in the optimal range
2.2) Feedback mechanism must indicate when patient is outside the optimal inhalation range
2.3) Feedback mechanism must record and time from the start of inhalation for a minimum of 4 seconds and a maximum of 6 seconds
Justification: Flow rate indicators allow for users to utilize the device in the most efficient way. Real time visual feedback help maintain the correct flow rate.
Must be universally adaptable to multiple commercially available valved holding chambers.
Specifications:
3.1) Must attach to the high flow alert port without modification to the existing VHC
3.2) The attachment must form a secure seal and prevent leaks greater than 10% at 1 PSI
Justification: Interfacing with the high flow port allows the device to measure inhalation without altering medication delivery
Must ensure precise and reliable flow measurements by maintaining an airtight connection with the High flow alert port.
Specifications:
4.1) The attachment must form a secure seal and prevent leaks greater than 10% at 1 PSI
4.2) The sensor must detect flow rate +/- 10% accuracy
Justification: Small leaks can distort flow rate readings. A proper seal improves sensor reliability making the the feedback system more effective.
All electronic components must meet electric safety standards IEC 60601-1
Specifications:
5.1) The total leakage current from the device should not exceeded 100uA
5.2) Must remain under a safe operating temperature range of 15 degrees Celsius to 40 degrees Celsius
Justification: These safety standards will ensure that the user of the device is not at risk of any for of injury from the electricals of the device component.
Must meet cleaning and disinfection regulation in accordance with ISO 17664
Specifications:
6.1) All components must be made of materials compatible with common disinfectants which include soaps and alcohol-based disinfectants
Justification: Adequate cleaning avoids contamination of medication and eliminates residue buildups.
Must maintain structural integrity and full functionality after undergoing a standard cleaning regime
Specifications:
7.1) Device must be able to withstand up to 50 cleaning cycles without degradation
7.2) The device must ensure that the delivered dosage is within 10% below the prescribed dose released from inhaler after cleaning
Justification: Medical devices in close contact with patients require frequent cleaning to meet hygiene control standards. This device is intended for long-term use; frequent cleaning should not compromise structure or functionality.
The device must be able to turn on and off and indicate whether its turned on or off.
Specifications:
8.1) The device must include an ‘On’ indicator that activates within 1 second of the device being turned on
8.2) The device’s power supply must ensure the device is operational for at least 10 working hours without needing to be replaced
Justification: A clear on/off indicator ensures users can confirm when the device is active and ready for use. Minimizes risk from potential electrical hazards and conserves battery life
————————————————————————————————————–
Standards
Standard #1
ISO 20072 – Aerosol drug delivery device design verification — Requirements and test methods, ensures device performance, efficacy, and safety.
Application to project: ISO 20072 will serve as a verification activity. This standard will be used to validate flow rate tests and the delivery of the aerosolized drug.
Citation – ISO 20072:2009. ISO. (2020c, March 9). https://www.iso.org/standard/41989.html
Standard #2
IEC 60601-1 – Medical Electrical Equipment – General Requirements for Basic Safety and Essential Performance. Specifies general safety and performance requirements. Focuses on electrical and mechanical hazards.
Application to project: IEC 60601-1 will ensure the overall electrical safety of the device. In order for the device to comply with this standard electrical and mechanical assessments will be performed. These tests will serve as additional verification activities.
Citation – IEC 60601-1-11:2015. ISO. (2020a, September 9). https://www.iso.org/standard/65529.html
Standard #3
ISO 23747 – Anaesthetic and respiratory equipment — Peak expiratory flow meters for the assessment of pulmonary function in spontaneously breathing humans. Outlines requirements for peak expiratory flow meters.
Application to project: ISO 23747 will ensure the accuracy, reliability, and safety of respiratory data measurement.
Citation – ISO 23747:2015. ISO. (2020b, December 3). https://www.iso.org/standard/64926.html
Standard #4
ISO 13485 – Medical Devices – Quality Management Systems. Ensures the device is designed and manufactured properly and that it meets regulatory requirements.
Application to project: ISO 13485 will guide the design and development process of the device. Overall, this standard will verify all processes regarding the design, development, and production of the device.
Citation – ISO 13485 – Medical Devices. ISO. (2020, March 11). https://www.iso.org/iso-13485-medical-devices.html
Standard #5
ISO 14971 – Application of Risk Management to Medical Devices. Highlights the risk management processes for medical devices, Focusing on design, development, and use of medical devices.
Application to project: ISO 14971 will guide the manufacturers of the device to identify potential hazards of the device, determine the probability of the device to undergo these hazards, and come up with different ways to mitigate the hazards from occurring and potentially injuring a user.
Citation – Recognized consensus standards: Medical Devices. accessdata.fda.gov. (n.d.). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfstandards/detail.cfm?standard__identification_no=41349
Standard #6
IEC 62304 – Medical device software — Software life cycle processes. Covers the development, maintenance, and management of software.
Application to project: IEC 62304 will ensure that the software of the spacer is developed and maintained while focusing on risk management and quality assurance. The software will be put through tests to ensure reliability and safety.
Citation – IEC 62304:2006. ISO. (2021, August 20). https://www.iso.org/standard/38421.html
Standard #7
IEC 60601-1-11 – Medical Electrical Equipment Used in the Home Healthcare Environment. Ensures the device is safe for at home use.
Application to project: IEC 60601-1-11 will guide the home safety feature of the device allowing for patients and caregivers to use the device outside of a clinical setting. Overall, the manufacturers will ensure that the device is safe to use in a non-clinical environment.
Citation – IEC 60601-1-11:2015. ISO. (2020a, September 9). https://www.iso.org/standard/65529.html