The hazard analysis was performed based on 7 severity effects that the patient might be exposed to when handling the device. The baseline effect is “No Ham,” it is assumed that the user will not be exposed to any harm when interacting with the device, a rating of 1 was assigned to this effect. The second severity effect taken into consideration is pressure marks since this can happen when the user is holding the device for a long period of time leading to discomfort. The rating assigned to this effect is 2 and has been labeled as “Negligible”. Minor Cuts and scratches from edges of the device have been rated as 3, and labeled as a “Minor” severity effect due to the fact that the user would not need to seek ambulatory treatment. Severe cuts, first-degree burns, and pain have been reported as a severity effect with a rating of 4 and labeled as “Critical”. Critical effects can be caused as a consequence of a short circuit within the device. Patients exposed to first degree-burns and severe cuts should see a healthcare provider or self treat. Device failure and malfunction have been taken into consideration with a rating of 5, and labeled as “Serious”. Malfunctioning of the device such as yielding wrong interpretation of test strips can cause unnecessary worry of the patients. Users can also be exposed to bacterial infection if the device is not properly sanitized before and after each use. This severity effect has been rated as “More than serious” and rated as 6 because the patient must seek ambulatory care and intake medications (Table 1).
The probability of occurrence illustrates how often the device can have a severe effect on the user. The occurrence of the device can be nearly impossible, very likely, occasionally, remote or frequent. Occasional is conceptualized as a severe effect occurring once every 50 tests taking in less than one month. Remote, when a severe effect happens once every five tests taken in less than one day. The occurrence probability is frequent if the device has a severe effect on the user during every test taken (Table 2).
Utilizing the parameters from the severity levels and probability of occurrence a risk priority number (RPN) table was created in order to evaluate the levels of risk under three different criteria. This criteria evaluates acceptable risk (R1), as low as reasonably practicable risk (R2) and unacceptable risk (R3) (Table 3).
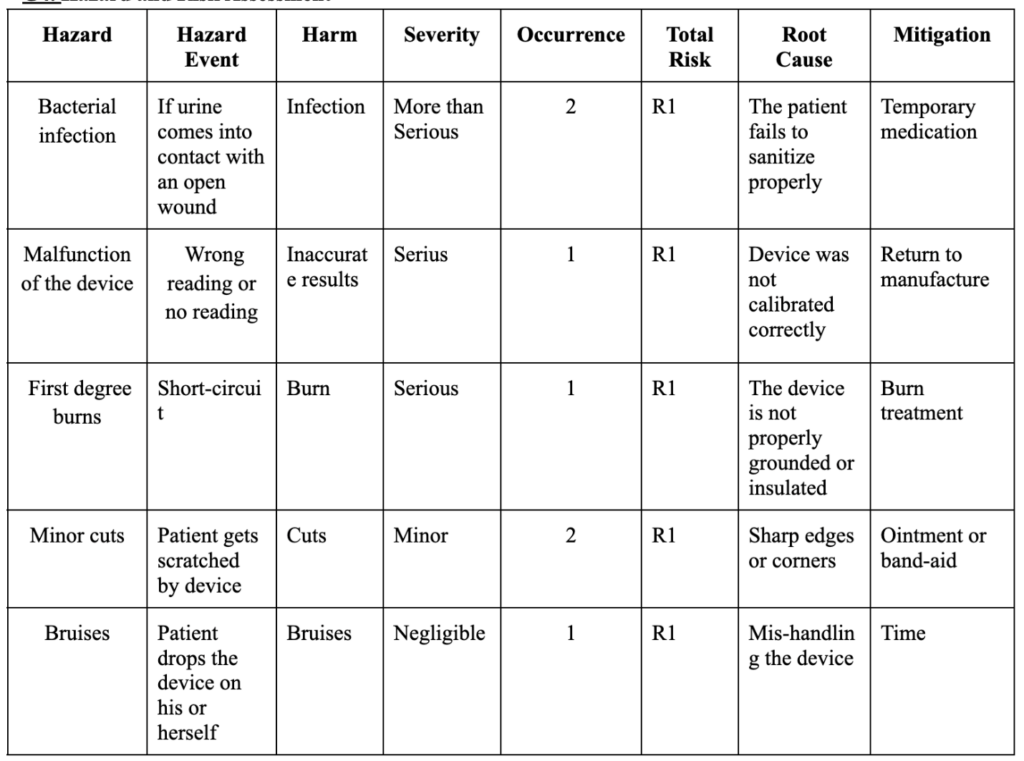
The Hazard risk assessment displayed an acceptable risk (R1) for all the possible hazards that can be caused during the user device interface, such as bruises, minor cuts, first-degree burns, bacterial infection and device malfunctioning (Table 4).
Table 1: Severity Table
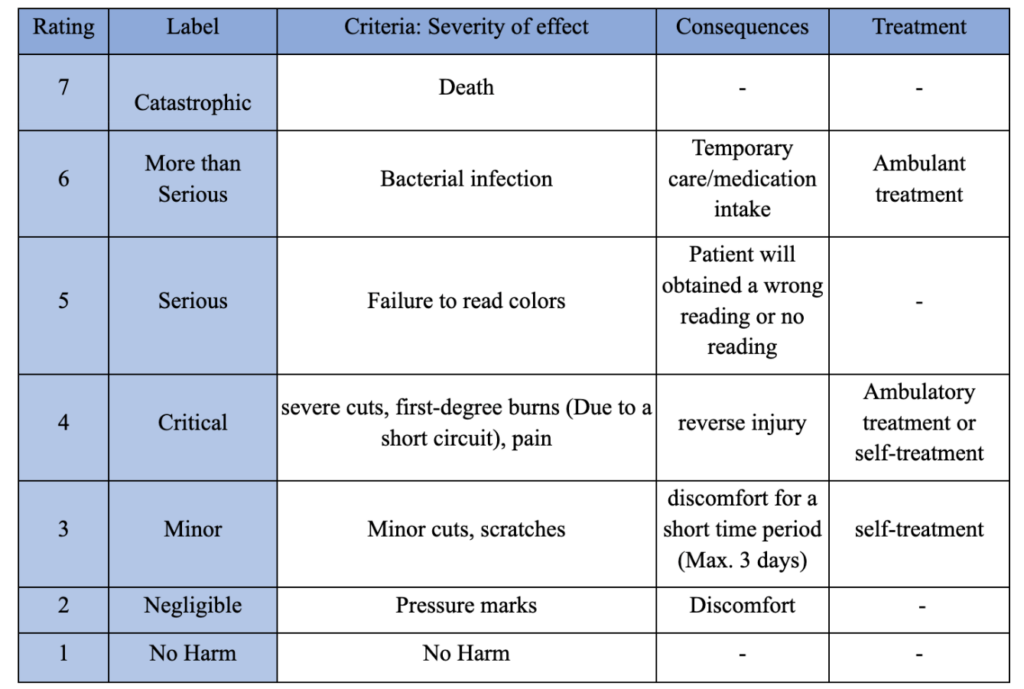
Table 2: Probability of Occurrence
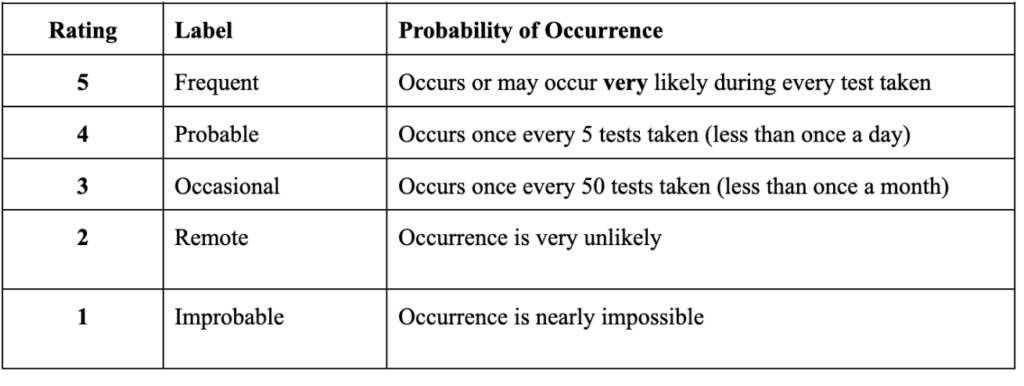
Table 3: RPN Table
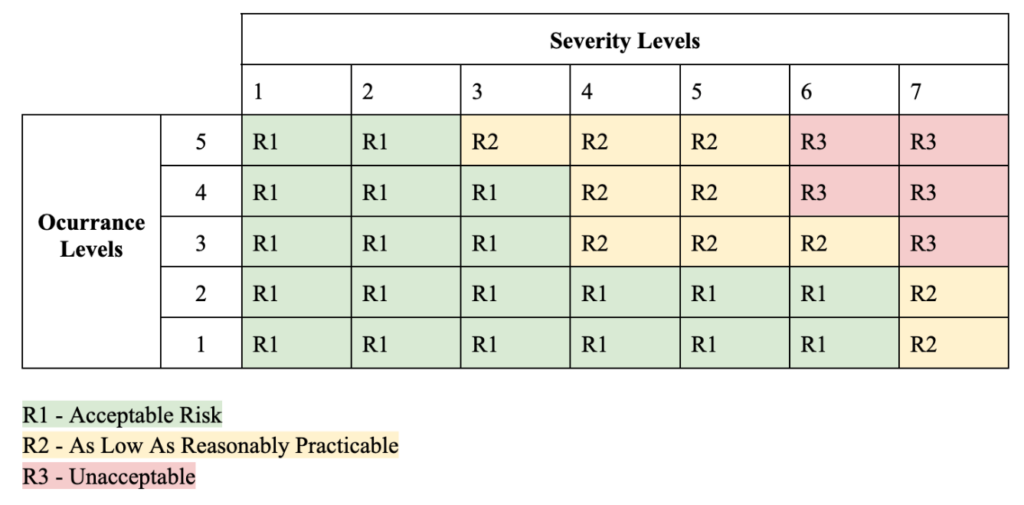
Table 4: Hazard and Risk Assessment