Device A – Patient Transfer Design Inputs
Device A – Design Input 1:
| Design Input | Verification Activity | Validation Activity | |
| Requirement | Specifications | Method / Protocol | Method / Protocol |
| A.1 – Device must transfer patients from surface to surface. |
A1.1 – The device lifts a patient up or down a maximum of 6 in. A1.2 – The device moves a patient a minimum of 3 ft laterally. A1.3 – The device moves the patient superiorly and inferiorly a maximum of 1 ft. |
A.1.1/A1.2/A1.3 – Specific distances will be measured during the transfer process. The minimum and maximum distances specified will not be exceeded during the 10 trials. |
A.1 – Patient survey assessing comfortability and sense of safety. |
A.1 – Reduces the risk of injury and improves safety of the patient. Allows the patient to move from one place to another when in need of medical treatment [1].
A1.1/A1.2/A1.3 – Patients may need to move from gurneys to hospital beds to imaging tables and may require multi-directional movement to reach final position.
Verification Testing
- Line Pull Capacity 1000 lbs
- Accessories included: Wire rope and hook
- Cable Length: 32 ft
Validation Testing
- 10 trials successfully having the winch pull 250 lbs a distance of 3 feet
Device A – Design Input 2
| Design Input | Verification Activity | Validation Activity | |
| Requirement | Specifications | Method / Protocol | Method / Protocol |
| A.2 – The device must reduce the number of nurses required to transfer a patient. |
A2.1 – Reduces the required number of nurses by two with a minimum staffing requirement of two nurses. |
A2.1 – Compare the number of nurses required for transfers with the device to the recommended number per OSHA standard. |
A.2 –Survey the patient transfer personnel on their ability to complete transfer. |
A.2 – OSHA standards require one nurse/transfer assistant per 35 lbs of patient. The average American is 185 lb (170 lb for women 200 lb for men) meaning the average transfer requires five nurses or assistants [2].
A2.1 – Device accounts for transferring a minimum of 70 lbs of weight. Requires usage of 2 nurses in order for the device to function properly.
Verification Testing
- Video of person being pulled across table
Validation Testing
- The number of nurses required for transfers decreased by 2 nurses.
Device A – Design Input 3
| Design Input | Verification Activity | Validation Activity | |
| Requirement | Specifications | Method / Protocol | Method / Protocol |
|
A.3 – The device must minimize patient movement. |
A3.1 – Maximum hip rotation of 10 degrees. A3.2 – Maximum hip flexion of 15 degrees. A3.3 – Maximum hip abduction of 15 degrees. |
A3.1/A3.2/A3.3 –Measure the specified angles with a goniometer during transfer. |
A.3 – Transfer the patients with device and survey them about how the feeling of their hips and the movement they had underwent during the transfer process. |
A.3 – Patients with broken hips are unable to stand, walk, or even move without experiencing intense pain [3].
A3.1/A3.2/A3.3 – To effectively diminish the patient’s pain, the angles of hip rotation, flexion, and extension should not exceed 1/10 of total normal range [4].
Verification Testing
| Trial # | Max Abduction (°) | Max Flexion (°) | Max Rotation (°) |
| 1 | 0 | 0 | 15 |
| 2 | 0 | 0 | 12 |
| 3 | 0 | 0 | 8 |
| 4 | 0 | 0 | 3 |
| 5 | 0 | 0 | 11 |
Validation Testing
- 5 trials with patients surveyed on stability of the hip and comfortability with an average rating of high.
Device A – Design Input 4
| Design Input | Verification Activity | Validation Activity | |
| Requirement | Specifications | Method / Protocol | Method / Protocol |
| A.4 – The device must support a wide range of individuals in the patient population. |
A4.1 – The device supports a range of patients up to 250 lbs. A4.2 – The device supports a range of individuals’ height up to 6’3”. |
A4.1 –Calibrated scale will be used to measure the weight of each participant. Observe the device for any deformation or failures during performance at maximum weight of 250 lbs. A4.2 – Calibrated device will be used to measure the height of each participant. Observe the mechanics of the device to conclude that it is accessible for the entire height range. |
A.4 – The device will be tested based on individuals close to one extreme of the population range for this device (95th percentile) as well as individuals below the given range. |
A.4 – Patients who experience broken hips can be male, female, young, old, tall, short, heavy, light weight.
A4.1/A4.2 – The 95th percentile male is 6’2” and 246 lbs [5].
Verification Testing
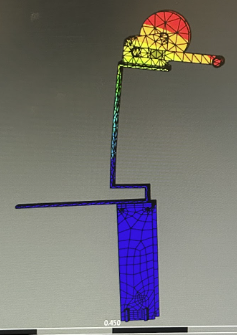
Validation Testing
- 7 patients were surveyed on the comfortability and safety while being transferred with an average rating of high.
Device A – Design Input 5
| Design Input | Verification Activity | Validation Activity | |
| Requirement | Specifications | Method / Protocol | Method / Protocol |
|
A.5 – The device must be sanitizable. |
A5.1 – The device will be sanitized using hospital grade disinfectant. |
A5.1 – Follow the protocol when using the hospital grade disinfectant to clean the patient transfer device. Observe the surface for residue |
A.5 – The performance of the device or any of its respective parts should remain the same after sanitizing it a minimum of 10 times using hospital grade disinfectant. |
A.5 – The device might be exposed to a number of bodily fluids during performance.
A5.1 – The CDC recommends that patient lifts and transfer device surfaces be cleaned with hospital grade disinfectant [6].
Verification Testing
- Surface was cleaned of machine shop residue using hospital-grade wipes, no residue remained.
Validation Testing
- The device’s performance remained the same after repetitive sanitization.
Device B – Transfer Bridge Design Inputs
Device B – Design Input 1
| Design Input | Verification Activity | Validation Activity | |
| Requirement | Specifications | Method / Protocol | Method / Protocol |
|
B.1 – The device must bridge the gap between a bed and transfer surface. |
B1.1 – The device should span a gap of 18 inches. |
B1.1 –The dimensions are verified on the dimension plans. |
B.1 – Survey patients on the comfortability of the transfer from the original surface, to the device, and to the final location. |
B.1 – There can be a multitude of surfaces patients are transferred across (hospital beds, hospital gurneys, and imaging tables).
B1.1 – The 2 proposed surfaces may have guard rails and other parts attached to the bed.
Verification Testing
Validation Testing
- 7 subjects were surveyed on the safety and comfortability during this process and rated the transfer a rating of high.
Device B – Design Input 2
| Design Input | Verification Activity | Validation Activity | |
| Requirement | Specifications | Method / Protocol | Method / Protocol |
|
B.2 – The device must be able to support the full weight of a person. |
B2.1 – The device should support a 250 lbs evenly distributed at each corner of the device. |
B2.1 – 250 lbs will be evenly distributed across 4 corners, and a stress test will be performed testing that the device will not deform. |
B.2 – Survey will be implemented asking the participants if they felt steady and secure while on the device. |
B.2 – There may be points at which the whole body rests only on the device.
B2.1 – The 95th percentile male is 246 lbs [5].
Verification Testing
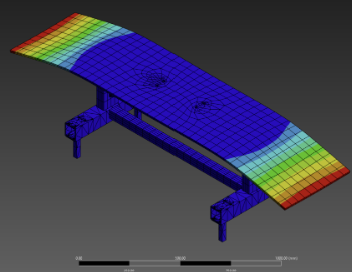
Validation Testing
- 7 subjects surveyed on comfortability and safety with an average rating of high.
