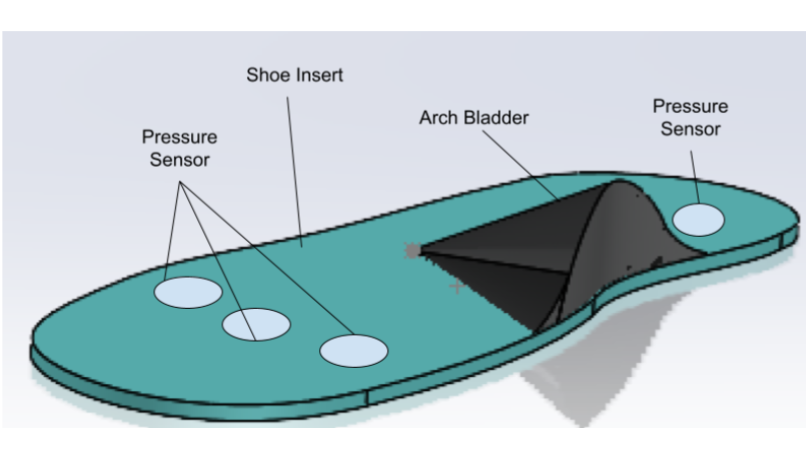
This page is dedicated to the initial outline of our device and the more detailed outline that will explain our design choices for our final prototype. At the start, our team began with an outline of what the device was going to do, figure 1.
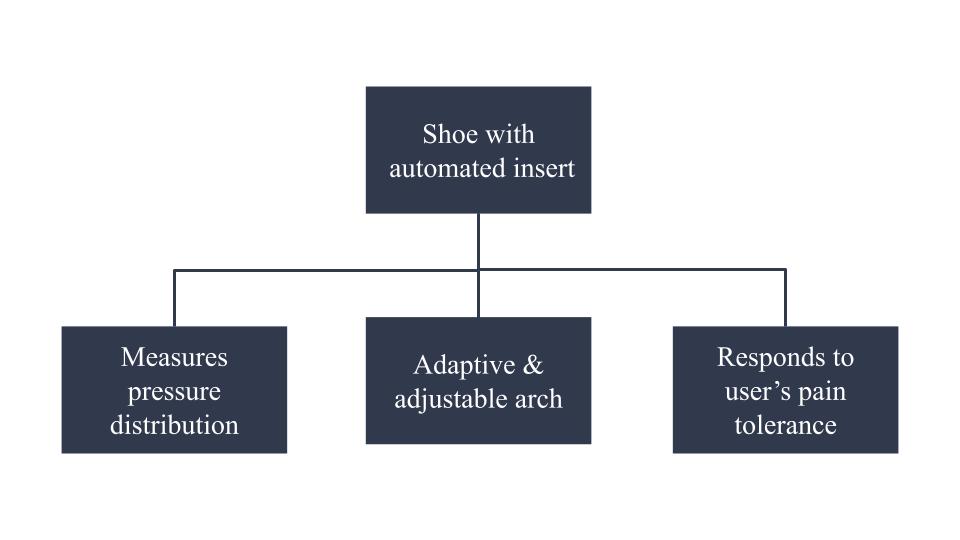
To explain in words, our team set out to create a device that addresses the need for an everyday shoe insert that changes arch height incrementally over time in harmony with the user’s pain tolerance. Further, more specific requirements were created to define what would verify that our team successfully made the device that was intended, table 1. After understanding what the device was required to do we moved from sketches to modeling the device using a computer-aided design program called SolidWorks, figure 2.
| Design Input | Verification Testing |
| 1. The device must be able to monitor the development of the arch structure over time. | a) Using weights and applying the range of specified forces to the measuring device. b)Using known body weight and standing on the pressure sensors. Calculating the percent of the body weight distributed between the sensors. c) Measuring the distance between the sensors and comparing it to the width of the region divided by the number of sensors being placed. |
| 2. The device must be adjustable to be able to change as the arch changes. | Measure peak arch height of the device at each of the 5 stages of incrementing using calipers. |
| 3. The device needs to withstand the activities of daily active living. | Add varying loading weights onto the device until creating max downward force under gravity and demonstrate that it still functions properly. |
| 4. Device in-use must allow for an individual’s normal walking gait. | a) Measure weight using a scale. b) Measure thickness using a caliper. |
| 5. The device must have sufficient battery power to operate for the period of time the user is using it. | Turn the device on for 10 hours every day and make sure it keeps operating for the specified time. |
| 6. The device must meet medical device electrical standards. | Measure currents using leakage current clamp meters and multimeters. |
| 7. The device must fit into podiatrist-approved shoe styles. | Measuring the insert and comparing it to the corresponding shoe size. |