DESIGN CONTROL
In compliance with the Food & Drug Administration (FDA) and Code of Federal Regulations (CFR) Title 21, formal procedure is to be followed during development of the P.H.A.M. device. Implementing these provided design control regulations of medical devices will establish a methodology to ensure the intended device requirements are met. The device input and output performance will be governed by the verification and validation processes to better guarantee the quality of the device and its performance.
The device requirements and specifications are put in place as supplemental guidelines to aid in our development procedure. Both the requirements and specifications are to coincide in order to build the physical device while also having it function properly.
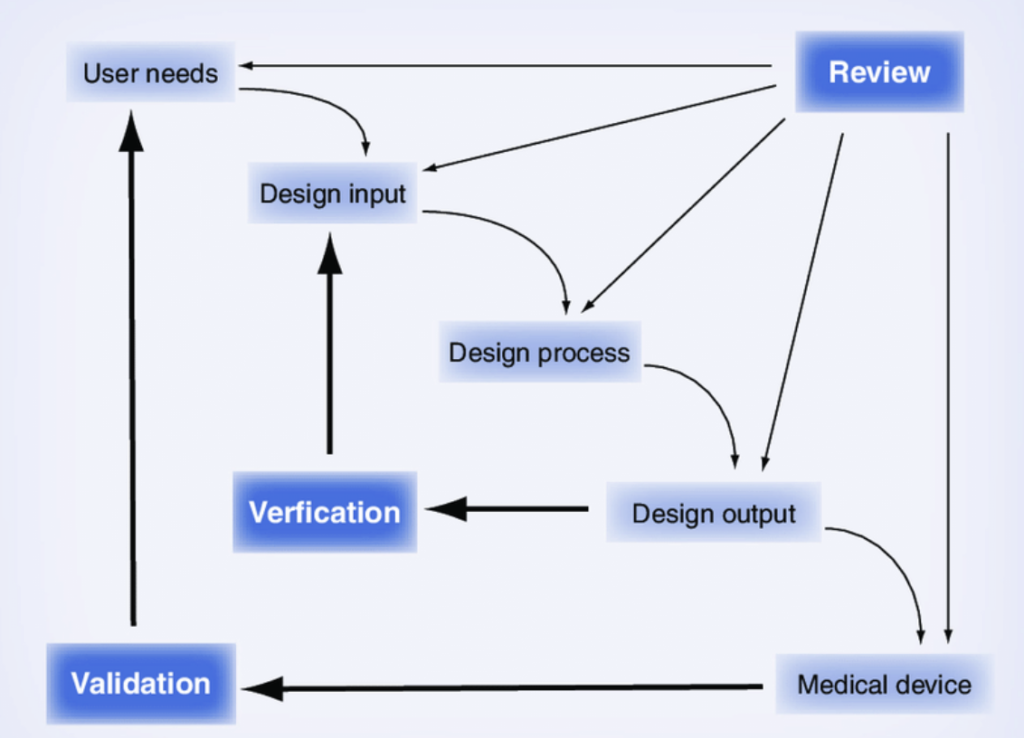
The common Waterfall Model, illustrating the relationship between design control components of medical device development.