September 8, 2018
The team is working to finalize the requirements & specifications of the device.
Requirements:
- Must include a handle shaped comfortably for the hand.
- Handle must allow for different grips.
- Must maintain an appropriate minimal weight for the surgeon to hold.
- Handle must allow for the attachment of a variety of accessories (i.e. blades).
- The handle must have a low vibrational frequency while the blade is rotating.
- Device must produce a rotational speed for the accessories to function.
- Handle must have a passage for irrigation.
- The device must be powered.
- The device must be safe.
- The device must be sterilizable.
September 21, 2018
The team received IRB approval for the Voice of Customer Survey.
October 1, 2018
The team is considering the following standards:
- Standards for use of equipment
- Standard for Orthopedic Devices, Surgical Devices
- Standard for Medical Electrical Equipment
- Standard for Care and Handling Orthopedic Instruments
- UL Standard for Safety Medical Electrical Equipment, Part 1: General Requirements for Safety
October 15, 2018
The team finalized the specifications for this project.
Requirement 1: Handle must be shaped comfortably for the hand.
Specification 1: The diameter of the handle must be between 30-50 mm. Justification: This is the recommended diameter for optimal flexibility and rotation of the instrument.
Specification 2: The device must be able to accommodate at least 2 different hand grips. Justification: Surgeon must be able to grip the handle at different positions.
Requirement 2: Device handle must have an acceptable weight for the surgeon to use.
Specification: The weight of the shaver should be less than 19.3 oz. Justification: Weight of the current Conmed Ergo hand piece is 19.3 oz and VOC indicates this as problematic.
Requirement 3: The handle must allow for reduced muscle fatigue on the surgeons’ hands when compared to current arthroscopic shaver systems.3
Specification 1: The handle should have a vibrational frequency below 8hz while in use. Justification: Human body is most sensitive to vibration in the frequency range of 1-8 hz.
Specification 2: Grip strength values before and after using device will not be significantly different. Justification: Reduction in grip strength is correlated with observed muscle fatigue.
Requirement 4: Device must produce a rotational speed for the accessories to function.
Specification: Blade must rotate at speeds of 100 – 12,000 RPM. Justification: This is the appropriate RPM range for arthroscopic shavers.
Requirement 5: The temperature observed on the surface in contact with the surgeon’s hand must not harm or cause discomfort.
Specification: The temperature measured on the external surface of the handle held by the surgeon must not exceed 43.8°C (110.8°C).
Justification: This is the heat pain threshold during contact with the hand.
Requirement 6: Handle must allow for the attachment of a variety of accessories (i.e. blades).
Specification 1: Joint for the blade must have a diameter between 3.5-6.0mm. Justification: The size of standard shaver blades of Ultracut, used by Conmed, is in this range.
Specification 2: Blades must fit with a latch mechanism that will transmit the functionality of the device.
Justification: Blades need to be securely attached to device.
Requirement 7 : Handle must have a passage for irrigation in which flow can be modulated.
Specification: The output volumetric flow rate must be +/- 10% of the input setting (between 0 and 1200 mL/min). Justification: Minimum and maximum flow rates for shaver suction function of ConMed 24k pump.
Requirement 8 : Surgeon must be able to control irrigation flow and blade operation.
Specification 1: When closed, incoming volumetric flow rate must be 0 mL/min. When open, the incoming volumetric flow rate must be +/- 10% the input volumetric flow rate provided by the pump.
Justification: The handle control must completely stop flow when needed, but not impede flow when control is not in use.
Specification 2: When controller is activated, blade rotation changes to the desired type (forward, backward, oscillation).
Justification: Surgeon must be able to quickly change blade direction based on use.
Additional Requirements & Specifications:
- Device must be powered.
- Device must meet the functional requirements of a sonic surgical instrument (21 CFR 888.4580)
- Instrument can cut bone.
- The device must be sterilizable.
- Device can function after being exposed to 100% humidity, 275ºF and pressure variations for up to 18 minutes.
- The device must be safe and follow electrical and health safety standards.
- All electrical equipment does not have any external defects prior to use (29 CFR 1910.334)
- The mains supply voltage must be at 110% of the highest-rated supply voltage and at the highest-rated supply frequency (IEC 60601-1)
- Typical leakage current values for Class II patient-care device is 150 µA (UL 60601-1)
October 24, 2018
The team cut into an old arthroscopic shaver to understand the internal structure & wiring. The team was unable to power the old shaver.
October 27, 2018
The team contacted Portescap – a company who makes motors for medical equipment such as arthroscopic shavers.
Novemeber 1, 2018
The team started designing. The VOC survey determined that weight was the least satisfactory feature of current devices. 50% of the responses identified muscle fatigue as an issue with using current bone shavers.
November 5, 2018
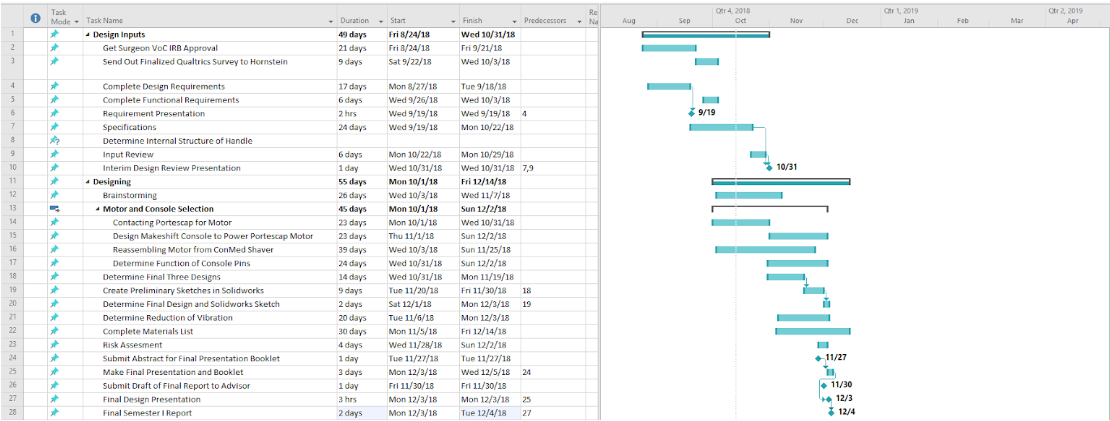
The team finished the Gantt Chart & budget. They are attached below.
November 20, 2018
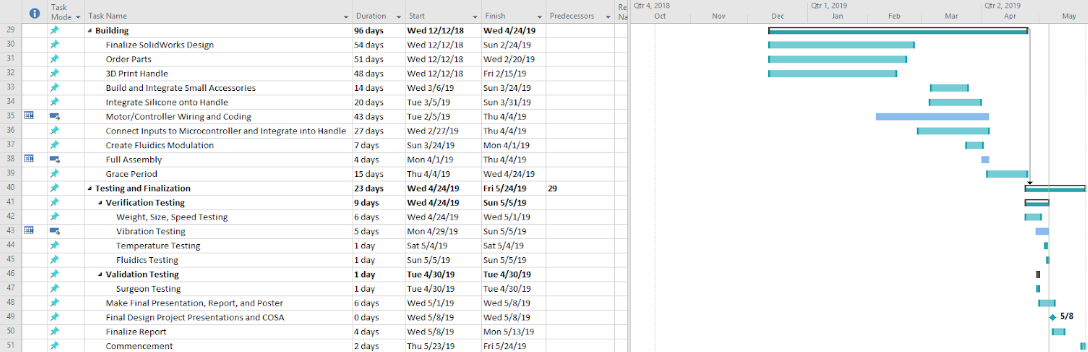
Team puts together sketches for designs. The team started Solidworks sketches.
Design 1:
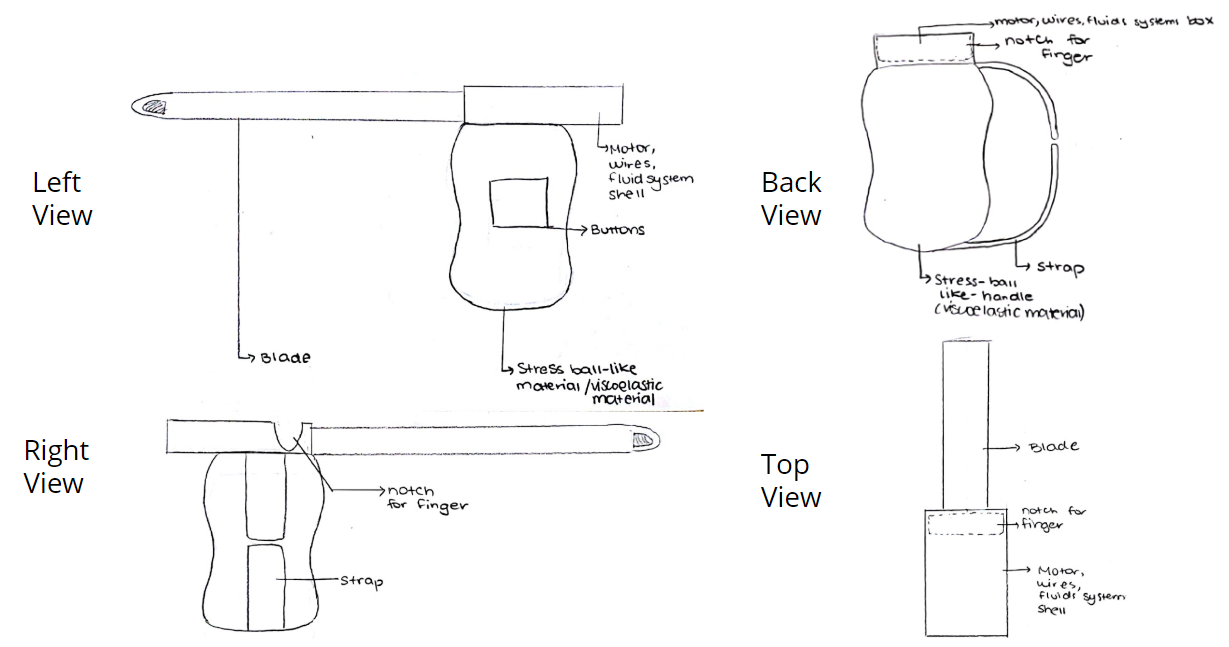
Design 2:
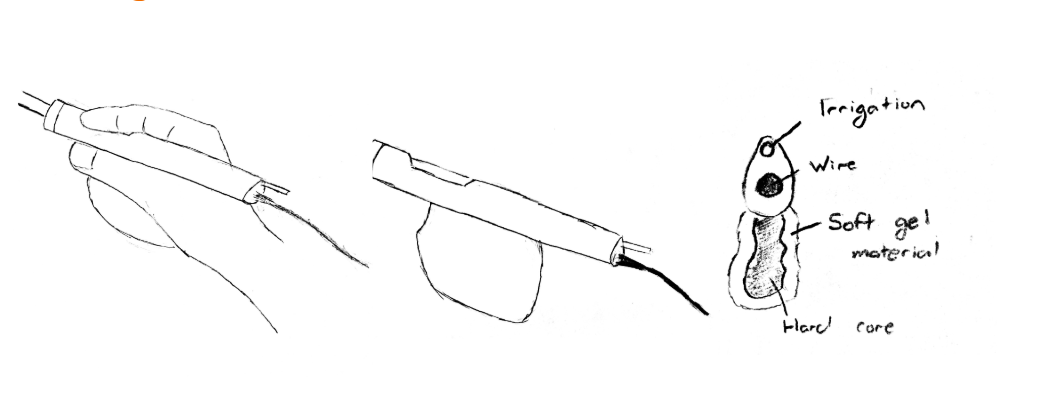
Design 3:
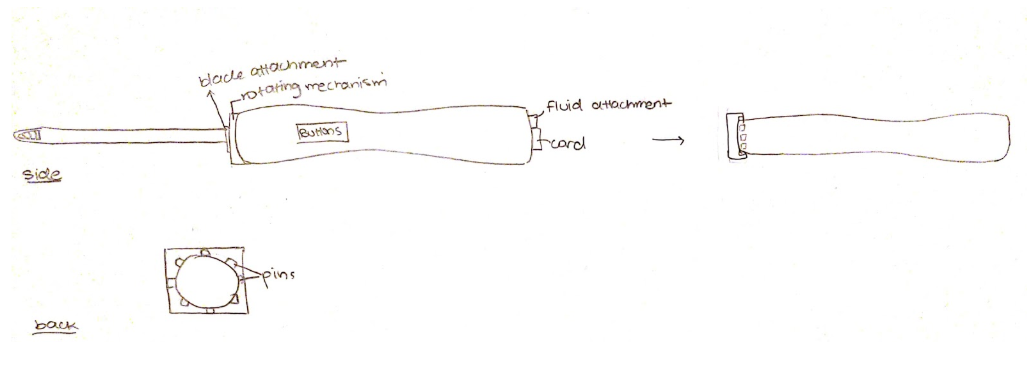
Design 4:
November 27. 2018
Team presented potential design solutions to surgeon consultant for feedback .
November 30, 2018
Team visited the Westchester, PA location of Portescap. Team was able to present design solutions to design engineers for feedback and was able to determine the motor for the project. Team worked with Portescap to power the motor and is able to implement skills learned into the project.
December 1, 2018
Team is determining material for encasing the motor as well as the viscoelastic material that will dampen vibration.
January 5, 2019
Team built clay models of possible design solutions and baked them with the appropriate weight.
January 20, 2019
Team went to the New Jersey Surgery Center to gain feedback on the clay models.
January 30, 2019
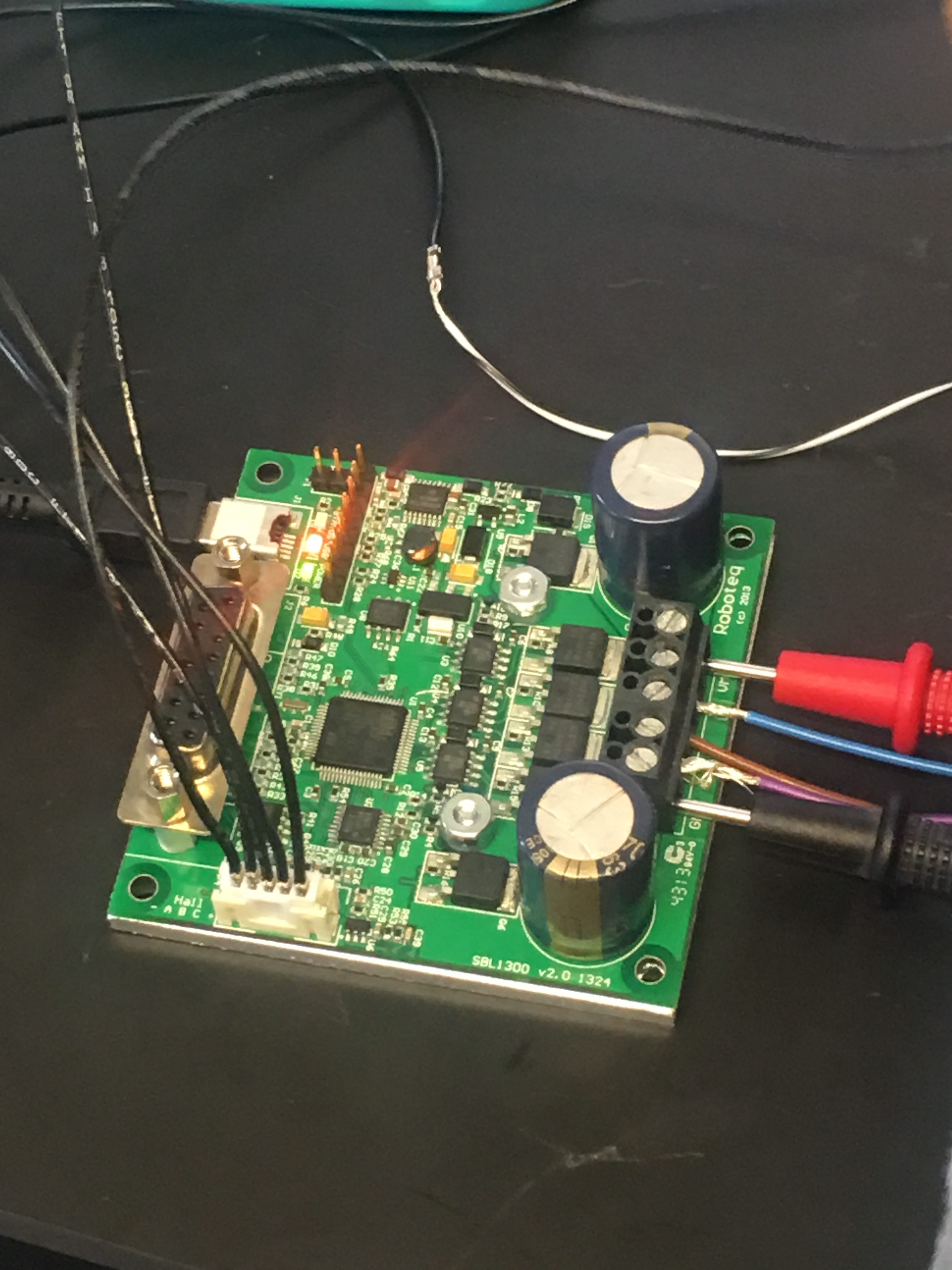
Team finished the electrical setup and was able to get the motor running in the forward/backward direction using the Roborun+ software as pictured below. They are also finalizing SolidWorks and getting ready to 3D print the prototype.
February 6, 2019
The team conducted risk assessment on their device, finished the post-device survey, determined the potting material, and started building the schematic for the printed circuit board. The team was also able to get the motor to oscillate using the Roborun+ software.
February 18, 2019
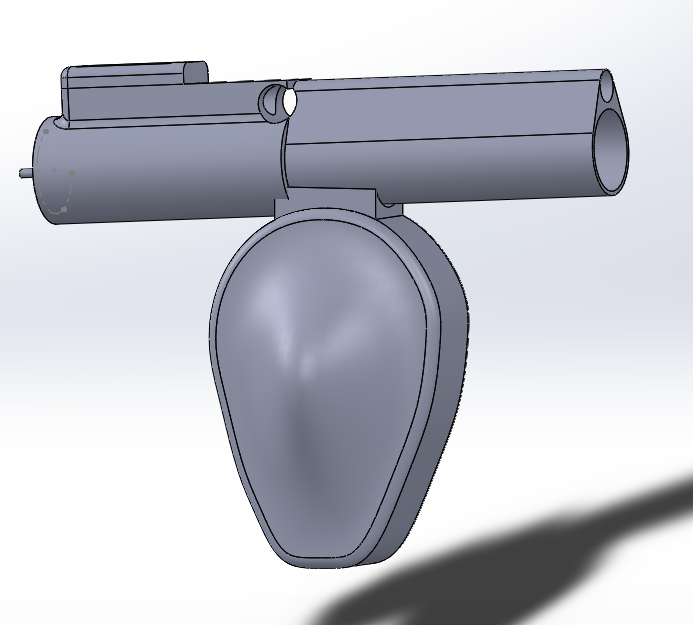
Team completed the verification & validation protocols and submitted the first draft of SolidWorks for 3D printing. Pictured below are the SolidWorks drawings of the shaver and the box that will hold the motor controller.
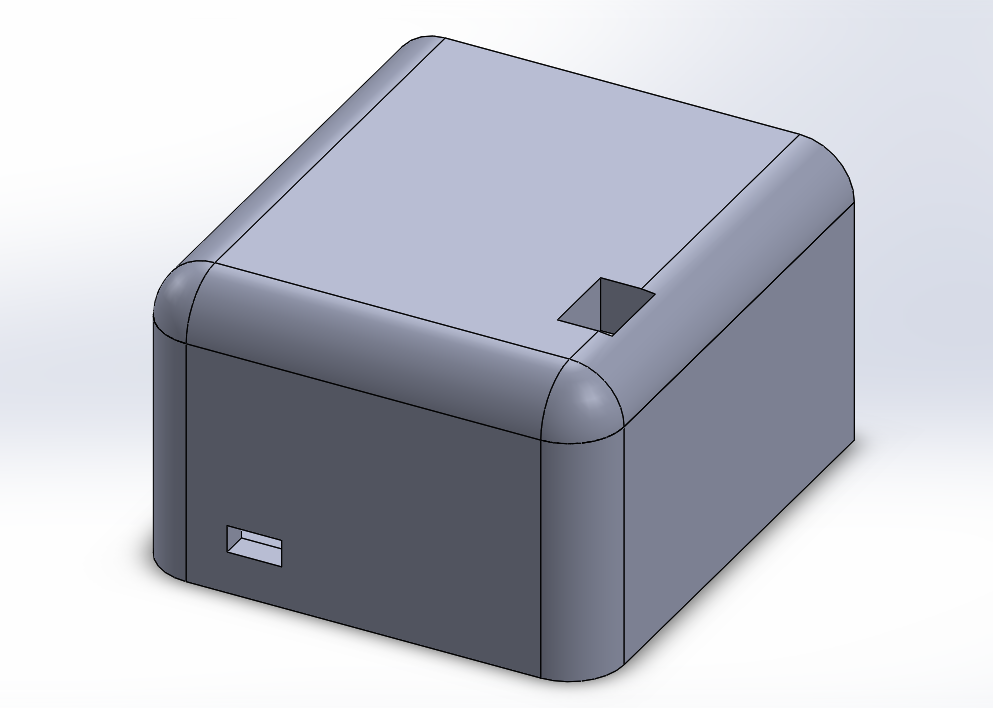
March 7, 2019
Team worked on the fluidics lever pins and fluidics passage that will be designed into the 3D printed prototype. They also began to incorporate button & potentiometer function into the device in an effort to control blade rotation and speed.
March 13, 2019
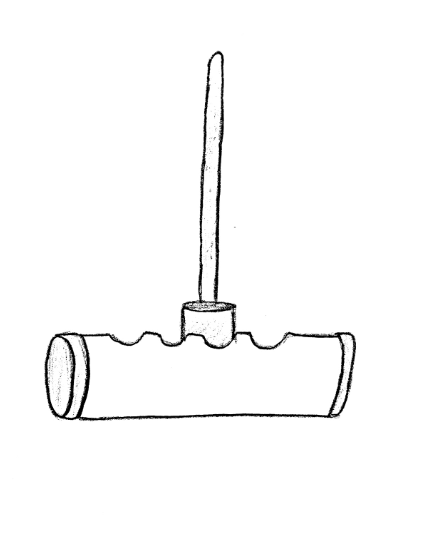
In order to connect the motor to the blade, a pin was designed in SolidWorks and fixed onto the top of the motor. Below are the SolidWorks drawing for the pin.
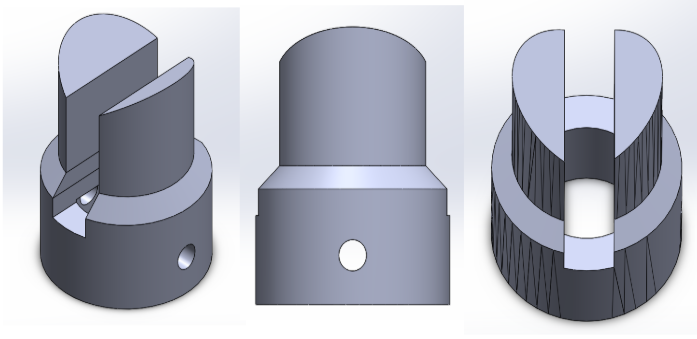

March 24, 2019
The team was able to program a button using the Roboteq computer interface and allowed it to control on/off function as well as counterclockwise, clockwise, and oscillatory direction. The button was inputted into the computer interface as an analog command and scripted to cycle through the different modes of blade direction. A potentiometer was also inputted into the microcontroller to control motor speed. The pictures below show where the button is place in our prototype. This placement proved to be a more convenient location compared to current shavers, since the user did not have to strain their hand to reach for it.
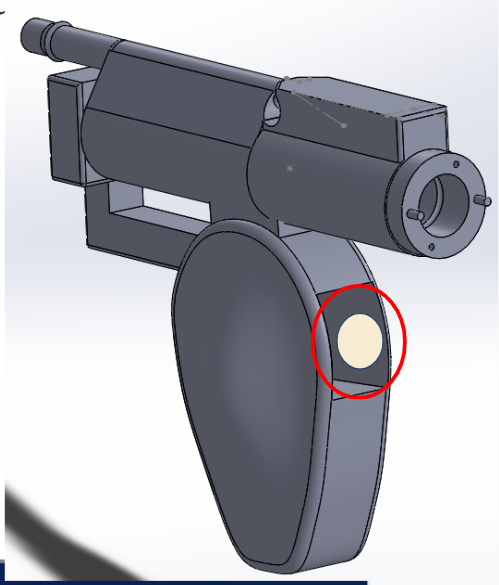
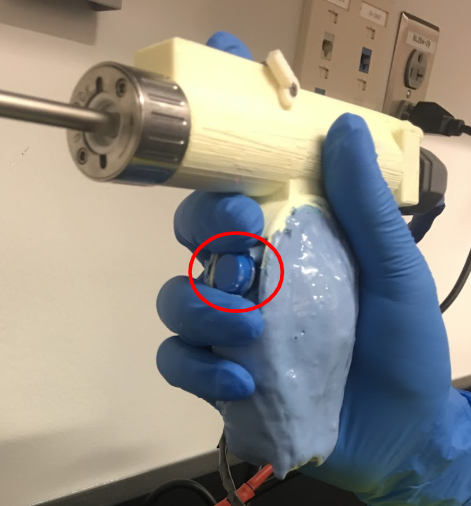
April 6, 2019
The team finalized all the SolidWorks by this date. The final prototype had a dome-like grip, a cylindrical tube on top that encased the motor, 3D printed fluidics lever pins, a passage for irrigation, a notch for the O ring to be inserted in order to waterproof the motor, and a passage for the button wires to weave through. The pictures below shows all the components of the printed handle and a cross-sectional view.
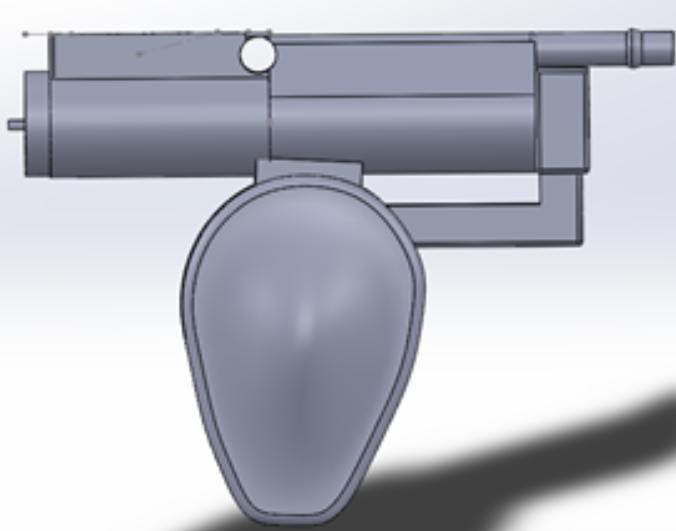
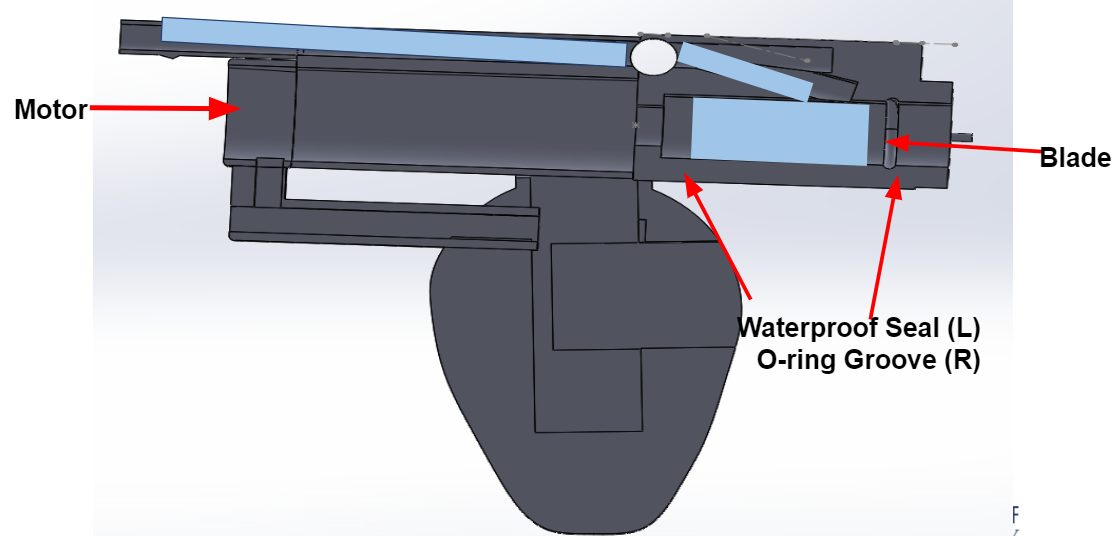
April 15, 2019
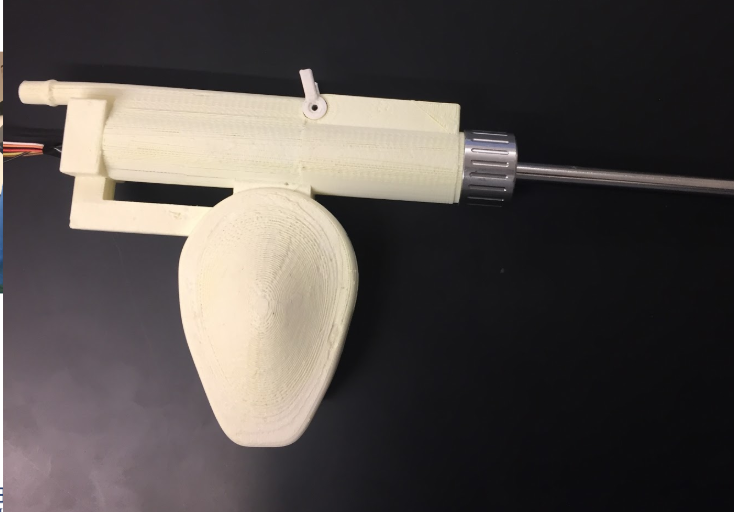
The team assembled the final protoype with all the components. A strain relief was added to the shaver, the wires were waterproofed, all wires were connected to the motor controller box, the motor pin attachment was inserted, the O ring was placed inside the handle, and the buttons and potentiometer were ready to be used. Lastly, the shaver was dipped in silicone. This is what our final assembly looks like! A schematic of all the components of our shaver system is also attached for better understatement.

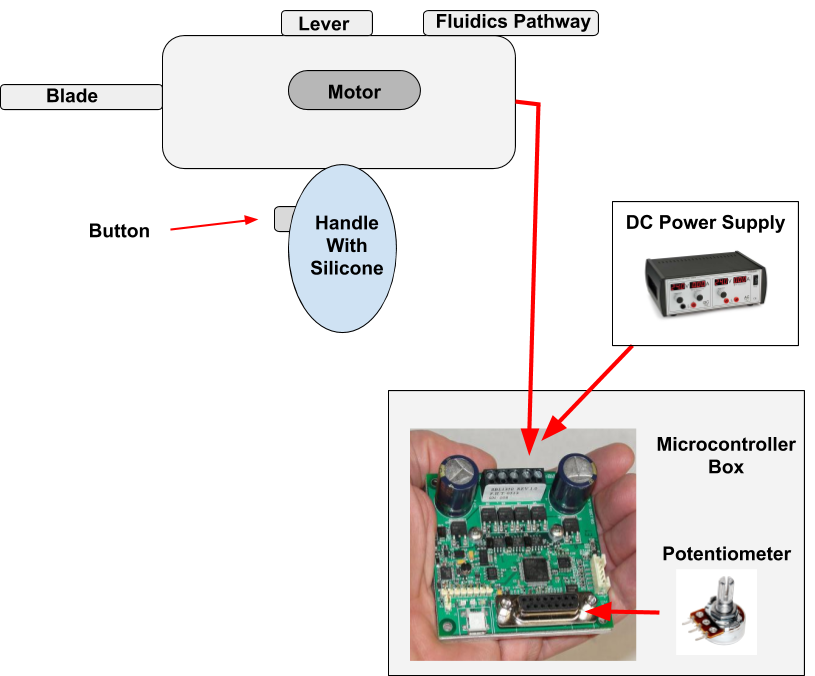
April 23, 2019
Once the final prototype was assembled, the team was able to conduct a mock surgery using the device on a pork shoulder. The device successfully cut a hole into the bone.
Click the link below to see a clip of the mock surgery!
May 1, 2019
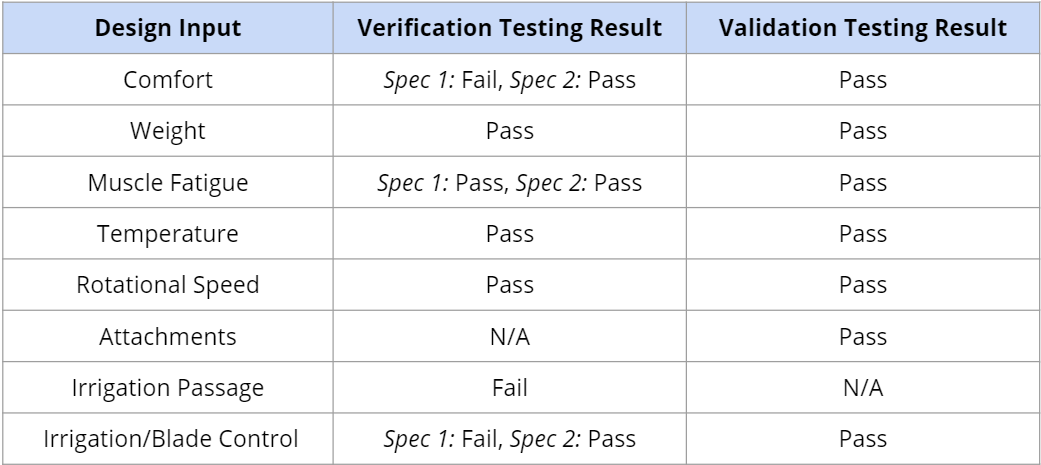
The team finished all verification and validation testing at this point. They were able to conduct muscle fatigue testing on orthopedic surgeons & surveyed them about the comfort-ability, weight, temperature, blade attachment, and muscle fatigue requirements.
Overall, vibrational acceleration was reduced compared to the ConMed Shaver. Muscle fatigue was also reduced after using the prototype compared to the muscle fatigue experienced after using the ConMed Shaver. The survey participants were all satisfied with the grip, temperature, blade function, and weight aspects of the device. They preferred the use of the prototype compared to the old shaver and noted there was apparent reduction in muscle fatigue.
After concluding all of the testing, the team was able to pass all validation testing. The only verification testing that were failed were the comfort verification due to a slightly bigger diameter and the irrigation verifications due to unexpected fluid leakage.
Here’s a summary of the results of the testing:
May 8, 2019
The team presented their final senior project presentations and presented at the Celebration of Student Achievement 2019 at The College of New Jersey.

