Week 4 (September 21st-September 28th)
During this week, our team came up with standards that our design must follow. Standards are rules, testing methods, recommended practices, and specifications. They are the force of law and is used to promote safety, ease of interfacing, uniformed performance, fairness, technological development, and professional development. Additionally, this week, we began to finalize the requirements for our design as well. Below is list of standards that our design must follow. This is not the final list and is subject to change in the near future:
| Standard | Justification |
|
F 1440-92(2008), “Standard Practice for Cyclic Fatigue Testing of Metallic Stemmed Hip Arthroplasty Femoral Components Without Torsion” https://ezproxy.tcnj.edu:2544/download/F1440-WITHDRAWN.8984.pdf Withdrawn bc information was updated in ISO 7206-4:2010 |
This standard will be used for us to validate our requirement regarding the length of time we want our implant to function. The testing requirements presented within this standard will be applied to any computational models used to assess our design’s endurance under cyclic loading. |
|
F 1612-95, “Standard Practice for Cyclic Fatigue Testing of Metallic Stemmed Hip Arthroplasty Femoral Components with Torsion” https://ezproxy.tcnj.edu:2544/download/F1612-WITHDRAWN.10725.pdf Withdrawn bc information was updated in ISO 7206-4:2010 |
This standard will be used to test fatigue of the hip implant and verify our specification of maintaining 2300N over five million cycles. The testing method outlined in the standard can be used to evaluate our model against others. This standard will be used for us to validate our requirement regarding the length of time we want our implant to function. The testing requirements presented within this standard will be applied to any computational models used to assess our design’s endurance under cyclic loading. |
| F 86-01, “Standard Practice for Surface Preparation and Marking of Metallic Surgical Implants” https://ezproxy.tcnj.edu:2544/download/F86.12837.pdf | This standard will be necessary when creating and designing our enhanced fixation of the femoral stem in hip implants. They are made of metals such as cobalt-chromium or titanium metals and must be prepared to be in patients in order to ensure that the body will continue to have normal biological processes. By following this standard, the risk of injuries to the body will decrease and allow for faster recovery post-surgery. |
| F 1814-15, “Standard Guide for Evaluating Modular Hip and Knee Joint Components” https://ezproxy.tcnj.edu:2544/download/F1814.14949.pdf | Allows surgeons to fit different sized components to match a patient’s anatomy (part between femoral stem and the head). Every patient is different and by following this standard, it gives surgeons the opportunity to decide the size of the hip implants, and also allows for more design opportunities as well. |
| F2033 − 12, “Standard Specification for Total Hip Joint Prosthesis and Hip Endoprosthesis Bearing Surfaces Made of Metallic, Ceramic, and Polymeric Materials” https://ezproxy.tcnj.edu:2544/download/F2033.30617.pdf | This standard will account for any of the non-metallic components that will be used within the newly designed prosthesis. |
|
F2996 – 20, “Standard Practice for Finite Element Analysis (FEA) of Non-Modular Metallic Orthopaedic Hip Femoral Stems” https://ezproxy.tcnj.edu:2544/download/F2996.21969.pdf |
In this project, the focus of the design is the femoral stem. Due to the fact that we will not be able to physically test our design, this standard will be utilized to analyze the metallic femoral stem. |
| ISO 17327-1:2018, Non-active surgical implants — Implant coating — Part 1: General requirements https://www.iso.org/standard/59591.html | It is a standard that all surgical implants must follow |
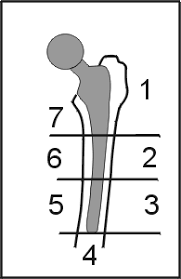
Additionally, our team was able to find a major research article that could potentially help us minimize stress shielding when creating our femoral stem. It shows through ANSYS analysis the stresses and strain that impacts different parts of the femur when the hip prosthesis is implanted. Our goal is to have our femoral stem implant look similar to the intact femur in the diagram below. The area that we will focus is called Gruen zone 1. The article can be found here: https://onlinelibrary.wiley.com/doi/full/10.1002/jor.23540
Week 5 (September 28th – October 5th)
Our team watched surgical procedures of hip implants to begin formulating potential designs. By watching these procedures, it gave us ideas of how to design the implant better to allow for an easier surgical operation. Not only that, the team began to discuss about possible materials to use for our prototyping to provide the best and accurate results.
Week 6 (October 5th-October 12)
In this week, our team was able to find a standard femur bone geometry to use in SolidWorks and ANSYS. We also discussed about our total budget and chose materials that similarly match the material properties of the femoral stem. In addition, we created a Gantt Chart and Budget Chart to keep ourselves organized and to efficiently complete tasks.
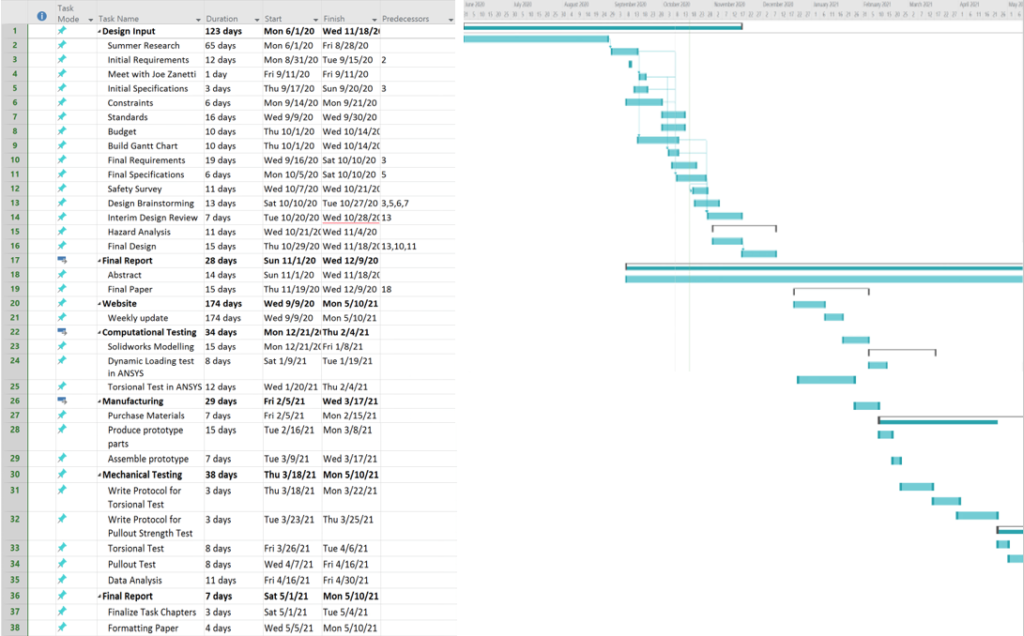
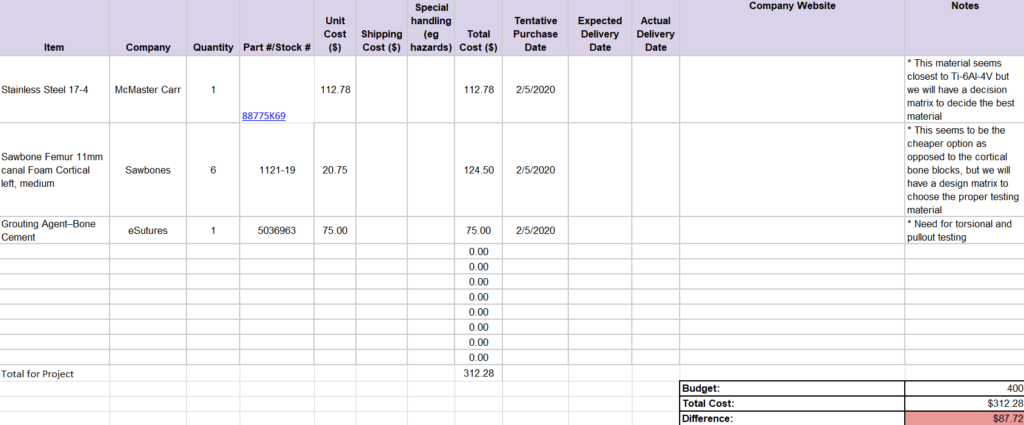
Below is the Gantt and Budget Chart: