*Please note the second and fifth design input no longer apply as our team went a different route in spring and decided to eliminate these criteria.
Design Input 1 |
Verification Activity |
|
|
Device Requirement |
Device Specification |
Method/Protocol |
|
1. Device must measure the compression pressure exerted through NGT insertion. |
1.1. Device must measure compression pressure ranged from 0 to 50 mmHg above 95% accuracy. |
1.1. Simulate compression pressures within 0 to 50 mmHg and ensure the device detects and measures these pressures above 95% accuracy. |
|
Justification |
||
|
Compression pressures of 32 mmHg or higher applied against soft tissues can cause damage.4 Users must learn how to avoid causing injury. Due to the training nature of this device, a 95% level of accuracy is acceptable and aligns with most medical devices.5
|
||
Design Input 2 |
Verification Activity |
|
|
Device Requirement |
Device Specification |
Method/Protocol |
|
2. Device must measure the shear stress exerted through NGT insertion. |
2.1. Device must measure shear stress ranged from 0 to 170 mmHg above 95% accuracy. |
2.1. Simulate shear stress within 0 to 170 mmHg and ensure the device detects and measures these shear stresses above 95% accuracy. |
|
Justification |
||
|
Shear stresses of 157 mmHg or higher applied against soft tissues can cause damage.6,7 Users must learn how to avoid causing injury. Due to the training nature of this device, a 95% level of accuracy is acceptable and aligns with most medical devices.5
|
||
Design Input 3 |
Verification Activity |
|
|
Device Requirement |
Device Specification |
Method/Protocol |
|
3. Device must measure the compression pressure exerted on anatomical sites most vulnerable to damage. |
3.1. Device must measure compression pressure values in 2 anatomical locations: the nasopharynx and the nasal cavity.8,9 |
3.1. Apply pressure on each individual sensor to examine independent activation of each component. |
|
Justification |
||
|
The nasopharynx and nasal cavity locations are most likely to be damaged during the NGT insertion process. The nasal mucosa in particular is sensitive to NTAPIs, and when pressure is exerted on the nasal mucosa of the nasal cavity and nasopharynx, there is an increased risk of NTAPIs. 8. Zhang, Y. B., Gou, L., Pei, J. H., Nan, R. L., Shen, X. P., Ge, L., Chen, H. X., Wang, X. L., Dou, X. M., & He, L. (2021). Development of a nomogram for predicting nasogastric tube-associated pressure injuries in intensive care unit patients. Journal of tissue viability, 30(3), 324–330. https://doi.org/10.1016/j.jtv.2021.06.008 |
||
Design Input 4 |
Verification Activity |
|
|
Device Requirement |
Device Specification |
Method/Protocol |
|
4. Device must inform the user if a compression pressure that would cause damage in a patient is being applied. |
4.1. If the compression pressure is above 32 mmHg, then device must inform user which sensor detects excess pressure. |
4.1. Simulate compression pressure at and above 32 mmHg and ensure the device accurately informs the user which sensor is experiencing excess compression. |
|
Justification |
||
|
User must be able to know when they have applied a compression pressure capable of damaging the patient’s tissues (32 mmHg or higher).4 This will also give the user an approximate idea of the NGT’s positioning and provide feedback to the user’s insertion technique. 4. Seyedhejazi, M., Hamidi, M., Sheikhzadeh, D., & Aliakbari Sharabiani, B. (2011). Nasogastric tube placement errors and complications in pediatric intensive care unit: a case report. Journal of cardiovascular and thoracic research, 3(4), 133–134. |
||
Design Input 5 |
Verification Activity |
|
|
Device Requirement |
Device Specification |
Method/Protocol |
|
5. Device must inform the user if a shear stress that would cause damage in a patient is being applied. |
5.1. If the shear stress applied is above 157 mmHg, then the device must inform the user which sensor is receiving that shear pressure. |
5.1. Simulate shear stress at and above 157 mmHg and ensure the device accurately informs the user which sensor is experiencing excess shear stress. |
|
Justification |
||
|
User must be able to know when they have applied enough shear stress to cause damage (157 mmHg) to the patient’s tissues.6,7 This will also give the user an approximate idea of the NGT’s positioning. Informing the user of an exerted pressure above this value provides feedback.
|
||
Design Input 6 |
Verification Activity |
|
|
Device Requirement |
Device Specification |
Method/Protocol |
|
6. Device must allow various NGT sizes and types to be utilized during training. |
6.1. Device must accommodate NGTs of 24 Fr (8 mm in diameter). |
6.1. Model will be measured using a caliper, with the thickness of the device subtracted from the measurement. |
|
Justification |
||
|
NGT sizes and types vary depending on anatomical differences among patients and the function required of the NGT. NGTs come in the following sizes: 8, 10, 12, 14, 16, 18, 22, and 24 Fr.11
|
||
Design Input 7 |
Verification Activity |
|
|
Device Requirement |
Device Specification |
Method/Protocol |
|
7. Device allows NGT to mimic passing through the nasal passage, pharynx, and esophagus. |
7.1. Device must align with the following anatomical dimensions indicated by medical imaging of a human: Nasopharynx: C.S.A. = 2.85 cm2 & length = 12.4 cm Esophagus: C.S.A. = 5.84 cm2 & length = 23-25 cm Nasal Valve: C.S.A. = 1.08 cm2 & length = 1.7 cm Nasal Conchae: C.S.A. = 1.95 cm2 & length = 1.92 cm 7.2. Device must be of silicone as that is the most accurate material for modeling the soft tissue interface.15,16 |
7.1. Device will be measured using a caliper, with the thickness of the device subtracted from the measurement and compared to literature values. 7.2. Material property tests will be conducted on our model to the values found in a study conducted by Payne et al.17 |
|
Justification |
||
|
User must be able to practice inserting the NGT without being hindered or experiencing resistance that does not align with clinical experience. If the NGT cannot pass through as it would in a patient, the training is no longer valuable. The dimensions specified above are based off of both females and males at an age of 53±11 years and BMI of 25.1.13
|
||
Design Input 8 |
Verification Activity |
|
|
Device Requirement |
Device Specification |
Method/Protocol |
|
8. Device must provide repetitive training opportunities for multiple users. |
8.1. Battery must be able to last 5 hours (or about 20 training sessions). 8.2. Exact battery capacity has yet to be determined. |
8.1. Device will be powered on for 5 hours and used for 15 minute intervals with 5 minutes of no use between them. |
|
Justification |
||
|
A training device is not useful if the method cannot be practiced with frequent repetition. The typical NGT insertion takes approximately 15 minutes.18 The device should provide the user consecutive training sessions without requiring re-charging. 18. Li, Q., Xie, J., Wu, J., Guo, R., Ma, W., Xu, G., Yang, M., & Deng, H. (2016). Novel Visual Nasogastric Tube Insertion System: A Feasibility and Efficiency Study in a Manikin. Gastroenterology research and practice, 2016, 7532172. |
||
Fall Semester Design Solutions
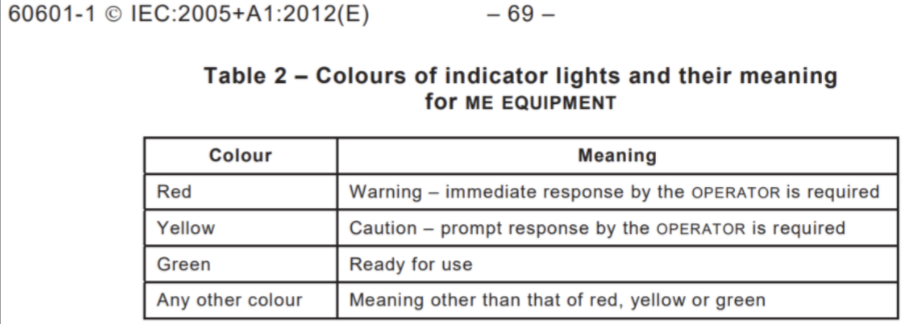
- Notification system will comply with IEC 60601-1 7.8.1 201210
- A light will indicate the severity, type of pressure, and location of excessive pressure on the device
Our device will follow these guidelines for indicating acceptable, cautionary, and unacceptable pressure during NGT insertion.4
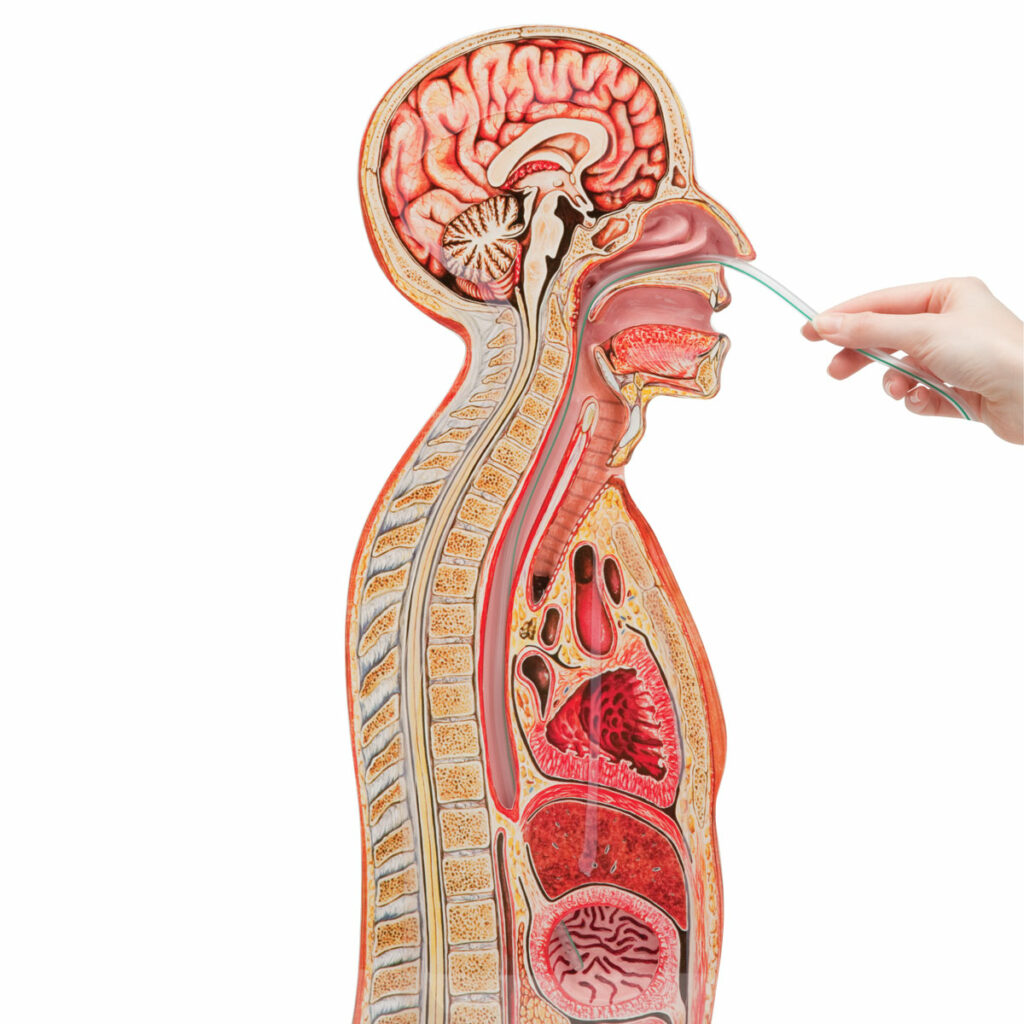
- Nasal passage, pharynx, and esophagus will be modeled based upon anatomical dimensions
- Either the anatomy will be directly 3D printed or a mold for the device itself will be printed
- May utilize pre-existing geometry models from patient scans and data
- Material of choice, silicone, will mimic qualities of soft tissues that compose the locations mentioned previously