These two weeks, the team has focused on initial design.
Our first idea for the valve is to use PyKC, a novel hydrogel. This hydrogel is insoluble in water and biological fluids. DMSO, frequently used for bladder flushing, could be used to degrade PyKC, making for a simple removal process when the device is no longer needed. As this is a novel material, more information is needed to finalize decisions going forward.
Our Second idea is to utilize a metal frame with a coating and a mesh within. The frame would be designed to have a ring of hollow tubes allowing it to expand and contract as needed. It would also be coated in a layer such as silicon dioxide to prevent cell growth.
For the catheter, we are planning to utilize a delivery mechanism similar to the procedure to implant ureteral stents. Via cytoscope, a guidewire is inserted into the urethra, through the bladder, and up the ureter. Once in position, the stent is fed along this wire with a catheter “pusher,” until the stent is in the proper position. At this point the guidewire and pusher are removed, leaving the stent behind.
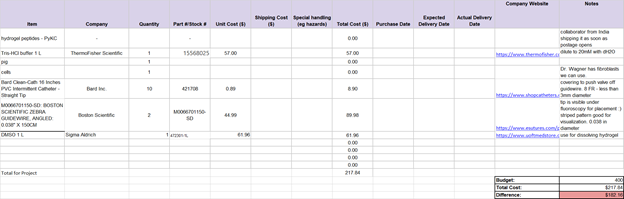
Additionally, the team has set up an initial budget and gaant chart to follow. Subject to change.
Budget
Gantt Chart – This is the schedule we anticipate to follow for the remaining duration of this project.

