These past two weeks, the team has conducted further research for design purposes.
As we learn more about the novel PyKC, we are leaning towards utilizing this design over the mechanical frame. The hydrogel meets our requirements for long-lasting(though degradability research is only available for 1 year), pH survivability, and water insolubility.
The hydrogel design can now be broken down into two more sub designs: stented and non-stented. The stented design would utilize a similar anchoring mechanism as cardiovascular stents. One downfall of this would be the inability to fully dissolve in DMSO, requiring a more invasive removal procedure, one of the reasons the mechanical valve was not selected. The other design would be a simple press-mold into the vesicoureteral junction made up solely of the hydrogel.
The team has been in touch with Dr. Debapratim Das of the Indian Institute of Technology for assistance in our utilization of the hydrogel. He has provided further information to answer our questions when needed, and has offered to provide the protein peptide base to make the hydrogel.
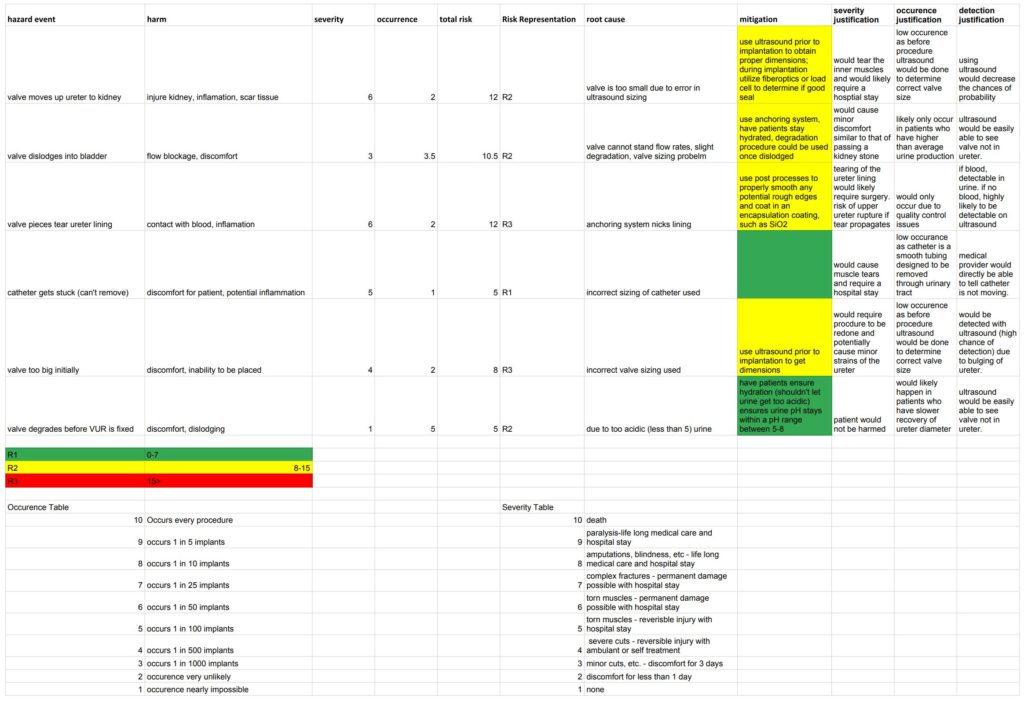
Additionally, the team has performed hazard analysis on foreseeable risks associated with the valve and delivery system. Hazard analysis is used to grade risk based on severity and occurrence. Additionally, we have included mitigation, or preventative measures, for risks assigned a risk representation of “R2.”