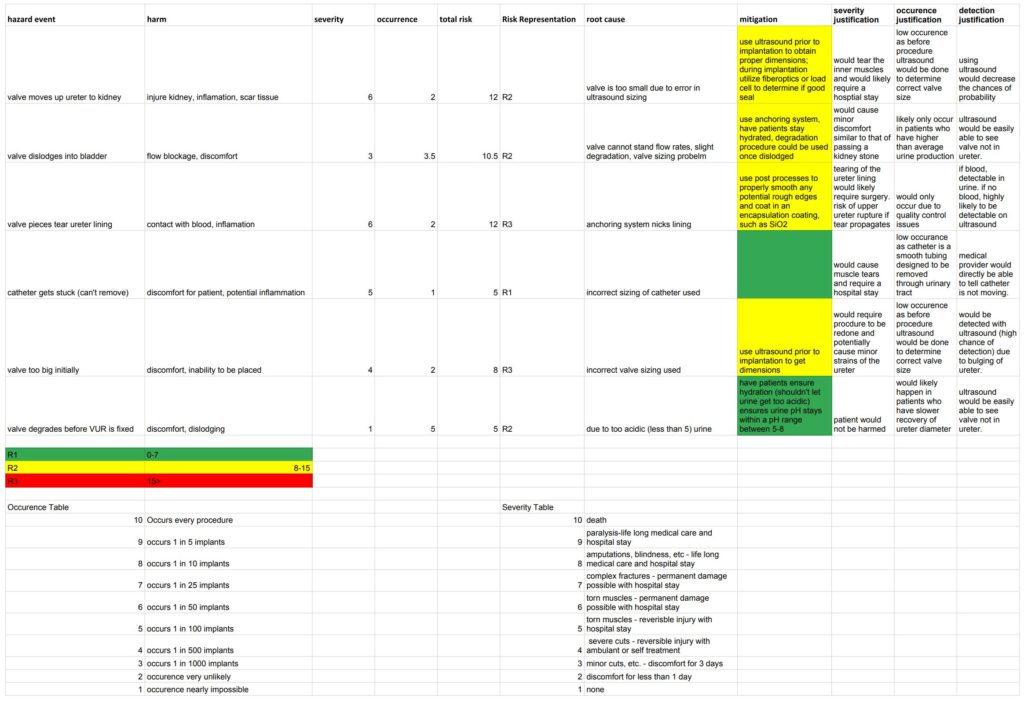
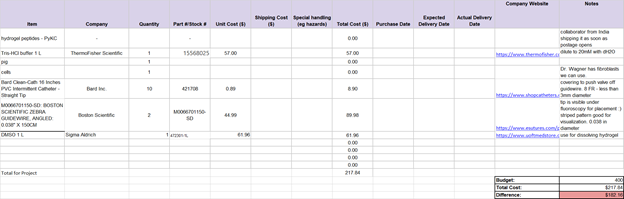
So far, the team has been discussing and finalizing requirements. Since this project consists of two main devices, an implantable valve and a catheter delivery mechanism, requirements were broken down as such and are as follows:
Valve Requirements
| Requirement |
Specification |
Justification |
|
1.) The device must remain intact while it is serving its purpose in the patient’s body.
|
1.1) Must be able to support a large range of pH present in urine (pH 5-8) [5].
|
1.1) pH changes can damage a material |
| |
1.2) Last at least 5 years [8]
|
1.2) In children, VUR often fixed within 5 years [8]
|
|
2.) The device must support normal urine flow into the bladder
|
2.1) The device must be able to support flow rates up to 25 mL/hour
|
2.1a) Flow rate is between 17.5-23.6 mL/hour [12]
|
|
|
|
2.1b) Urine flow is pulsatile/dynamic flow, not just steady state [12].
|
|
|
2.2) The device must be able to support intra-abdominal pressure up to 20 mmHg
|
2.2) Intra-abdominal pressure up to 5 mmHg is normal in adults, but with conditions such as obesity, it can get as high as 15 mmHg. Above 20 mmHg is associated with organ dysfunction [14].
|
|
3.) The device must “grow” with a child if a long-term fixture
|
3.1) The material needs to be able to elastically strain 233% to change with the ureter.
|
3.1) The ureter starts at 3-5 mm in healthy kids, greater than 7 mm in kids with VUR [15]
|
|
4.) The device must be biocompatible
|
|
4.) For any medical device approved by the FDA, biocompatibility testing must be doe following ISO 10993-1 [1].
|
|
5.) The device must stay in place
|
|
5.) Movement of the valve will initiate an immune response [4]
|
Catheter Requirements
| Requirement |
Specification |
Justification |
|
1.) The device must be able to reach the vesicoureteral junction.
|
1.1) The placement device must be able to range in length from 2 cm to 30 cm to accommodate both males and females, children and adults.
|
1.1a) The adult male urethra ranges from 15-30 cm long [2].
|
| |
1.1b) The average female child’s urethra length is 2.59 cm [3]
|
|
|
2.)The device must be able to fit into the urethra.
|
2.1) The catheter tubing must be less than 3 mm in diameter.
|
2.1) In children, the average diameter of the urethra is 3-4 mm [9].
|
|
3.1) The device must be able to place the valve correctly, and then be separated from the valve for removal from the body.
|
3.1) Once placed, the valve does not move more than 2 mm once separated from the catheter.
|
3.1a) This is the function of the device, as a catheter long-term would be uncomfortable for a child.
|
| |
3.1b) If the valve moves during placement, it needs to be close enough to still support the natural flap.
|
|
|
4.) The device must be biocompatible.
|
|
4.) For any medical device approved by the FDA, biocompatibility testing must be doe following ISO 10993-1 [1].
|
Additional Requirements
| Requirement |
Justification |
|
1.) The delivery of the devices must be simple and does not require additional surgical training.
|
1.) The goal of the company is to make the device usable internationally, especially in areas with fewer medical professionals.
|
|
2.) The cost of the procedure must be equivalent of already existing treatments
|
2.) The goal of the company is to ensure that insurance companies cover most of the cost.
|
|
3.) The devices must be manufactured at TCNJ
|
3.) Rules of senior project
|
|
4.) The devices must be as environmentally friendly as possible
|
4.) The goal of the company is to ensure a low carbon footprint, which can be done through the use of recycled materials in packaging
|
References
[1] FDA, Special Considerations – Biocompatibility. 05/22/2020. https://www.fda.gov/medical-devices/premarket-notification-510k/special-considerations#bio
[2]Kohler, Tobias S et al. “The length of the male urethra.” International braz j urol : official journal of the Brazilian Society of Urology vol. 34,4 (2008): 451-4; discussion 455-6. doi:10.1590/s1677-55382008000400007
[3]Halleran, Devin R., et al. “Urethral Length in Female Infants and Its Relevance in the Repair of Cloaca.” Journal of Pediatric Surgery, vol. 54, no. 2, 2019, pp. 303–306., doi:10.1016/j.jpedsurg.2018.10.094.
[4] “Recommendations.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 5 Nov. 2015, www.cdc.gov/infectioncontrol/guidelines/cauti/recommendations.html.
[5] Feher, Joseph. Quantitative Human Physiology. Elsevier. 2nd Edition. 2011
[6] Burr, RG and Nuseibeh IM. Urinary catheter blockage depends on urine pH, calcium, and rate of flow. Spinal Cord. 35: 521-525. 1997. https://www.nature.com/articles/3100424
[7] Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 17(2): 103-114. 1996. https://www.sciencedirect.com/science/article/pii/0142961296857553
[8] (https://www.hopkinsmedicine.org/health/conditions-and-diseases/vesicoureteral-refluxvur#:~:text=Most%20children%20who%20have%20grade,Surgical%20treatment%20is%20also%20available.)
[9] Chow, Jeanne S., and Annemieke S. Littooij. “Urogenital Pathologies in Children Revisited.” SpringerLink, Springer, Cham, 1 Jan. 1970, link.springer.com/chapter/10.1007/978-3-319-75019-4_7.
[10] Kakizaki H;Moriya K;Ameda K;Shibata T;Tanaka H;Koyanagi T; “Diameter of the External Urethral Sphincter as a Predictor of Detrusor-Sphincter Incoordination in Children: Comparative Study of Voiding Cystourethrography.” The Journal of Urology, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/12544337/.
[11] B;, Reinking LN;Schmidt-Nielsen. “Peristaltic Flow of Urine in the Renal Capillary Collecting Ducts of Hamsters.” Kidney International, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/7300113/.
[12] Kim, Kyung-Wuk, et al. “Analysis of Urine Flow in Three Different Ureter Models.” Computational and Mathematical Methods in Medicine, Hindawi, 4 June 2017, www.hindawi.com/journals/cmmm/2017/5172641/.
[13] Rollino, Cristiana, et al. “Vesicoureteral Reflux in Adults.” Giornale Italiano Di Nefrologia : Organo Ufficiale Della Societa Italiana Di Nefrologia, U.S. National Library of Medicine, 2011, www.ncbi.nlm.nih.gov/pubmed/22167611.
[14] Milanesi, Rafaela, and Rita Catalina Aquino Caregnato. “Intra-Abdominal Pressure: an Integrative Review.” Einstein (Sao Paulo, Brazil), Instituto Israelita De Ensino e Pesquisa Albert Einstein, 2016, www.ncbi.nlm.nih.gov/pmc/articles/PMC5234758/.
[15] “Megaureter & Ureter.” Cleveland Clinic, my.clevelandclinic.org/health/diseases/16305-megaureter.